Origlia Nicola
 Senior Research scientist
Senior Research scientist
Via Giuseppe Moruzzi, 1
56124 - Pisa
Tel 050-3153193
Fax 0503153210
This email address is being protected from spambots. You need JavaScript enabled to view it.
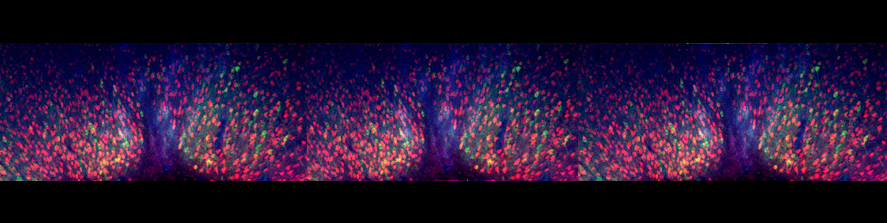
The entorhinal cortex physiology and its vulnerability to neurodegeneration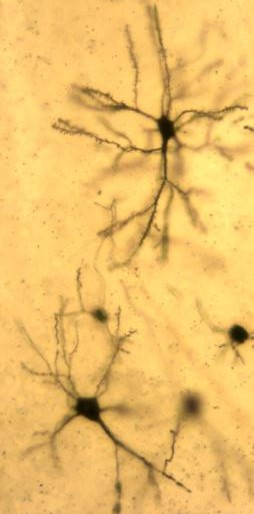
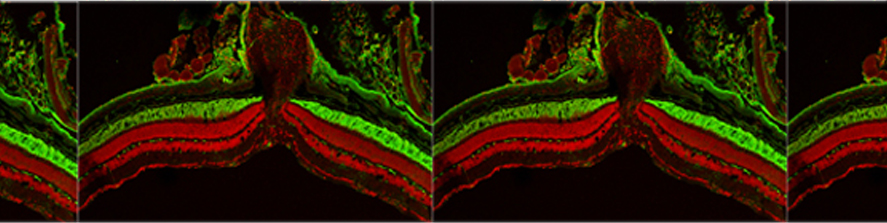
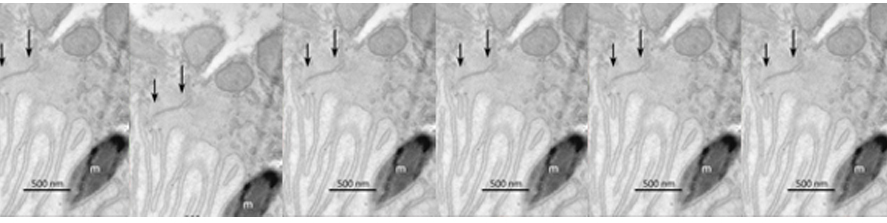
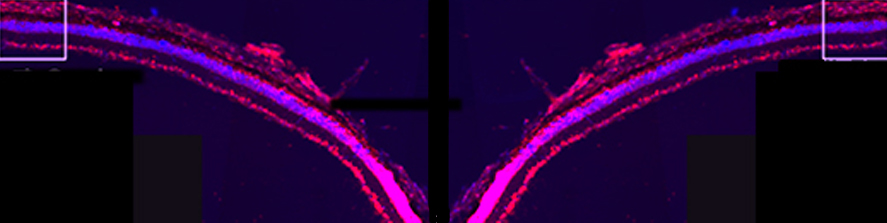
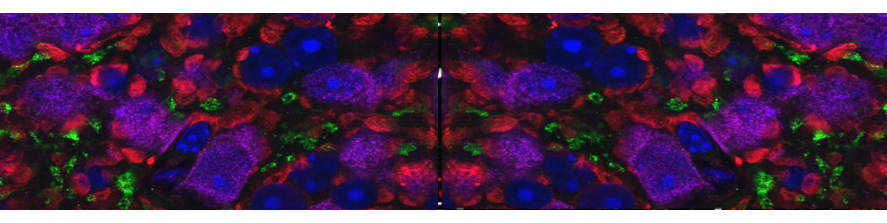
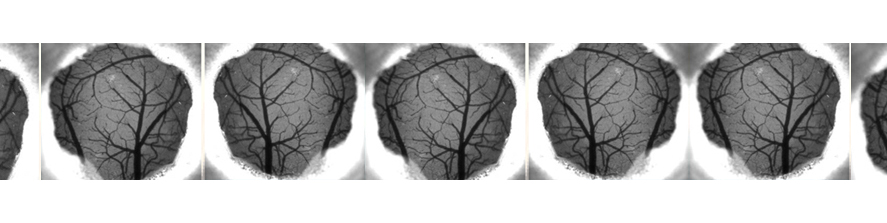
The main focus of the lab is to investigate factors and mechanisms regulating synaptic transmission and plasticity either under physiological conditions or in animal models of neurodegeneration. The experimental approach is based on electrophysiology, either in brain slices or in vivo. Combining electrophysiology with behavioral and molecular analysis, we identified key neurobiological mechanisms relevant for the synaptic impairment associated with neurodegenerative processes.The Entorhinal cortex (EC) is a parahippocampal region involved in learning and memory and exhibiting an high degree of plasticity. The EC is also central to the pathophysiology of Alzheimer’s disease (AD). The distribution of the typical AD hallmarks follows a specific progression, first beginning in layer II of the EC and then spreading to the hippocampal formation. The selective vulnerability of EC neurons might be the result of a particular susceptibility to microenvironmental factors, such as increased Aβ levels and vascular injury, that interact with molecular peculiarities of these cells.
Research’s Topics :
Lateral entorhinal cortex in episodic-like memory formation and recall
Representative publications
A Complete List of Published Work in My Bibliography:
https://www.ncbi.nlm.nih.gov/myncbi/nicola.origlia.1/bibliography/public/
Selected papers
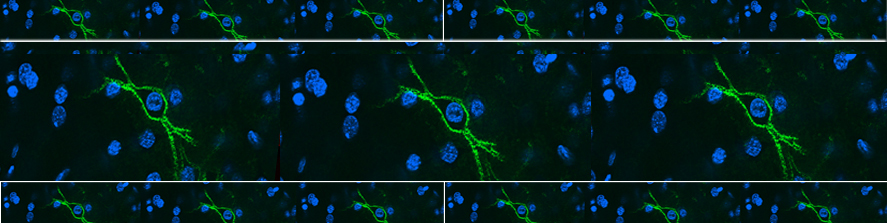
Tozzi F, Guglielmo S, Paraciani C, van den Oever MC, Mainardi M, Cattaneo A, Origlia N. Involvement of a lateral entorhinal cortex engram in episodic-like memory recall. Cell Rep. 2024 Sep 25;43(10):114795. doi: 10.1016/j.celrep.2024.114795
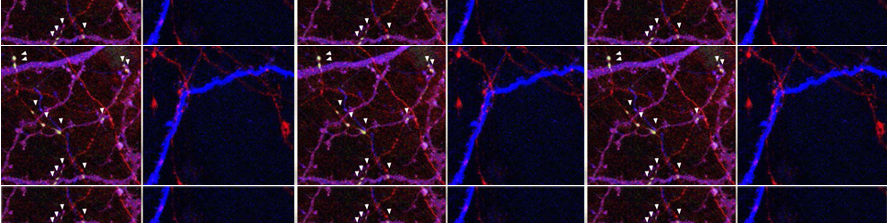
Falcicchia C, Tozzi F, Gabrielli M, Amoretti S, Masini G, Nardi G, Guglielmo S, Ratto GM, Arancio O, Verderio C, Origlia N. Microglial extracellular vesicles induce Alzheimer's disease-related cortico-hippocampal network dysfunction. Brain Commun. 2023;5(3):fcad170. doi: 10.1093/braincomms/fcad170
Gabrielli M, Prada I, Joshi P, Falcicchia C, D'Arrigo G, Rutigliano G, Battocchio E, Zenatelli R, Tozzi F, Radeghieri A, Arancio O, Origlia N*, Verderio C*. Microglial large extracellular vesicles propagate early synaptic dysfunction in Alzheimer's disease. Brain. 2022 Aug 27;145(8):2849-2868. doi: 10.1093/brain/awac083.
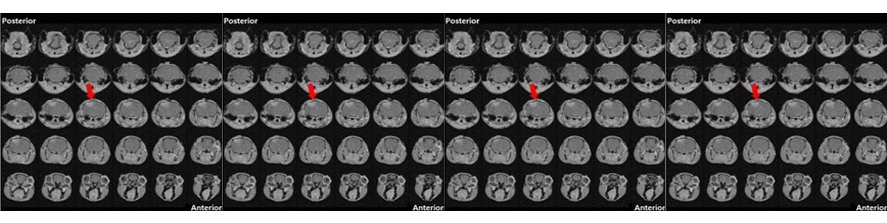
Tozzi F, Rutigliano G, Borsò M, Falcicchia C, Zucchi R, Origlia N. T(1)AM-TAAR1 signalling protects against OGD-induced synaptic dysfunction in the entorhinal cortex. Neurobiol Dis. 2021 Apr;151:105271. doi: 10.1016/j.nbd.2021.105271.
Accorroni A, Rutigliano G, Sabatini M, Frascarelli S, Borsò M, Novelli E, Bandini L, Ghelardoni S, Saba A, Zucchi R, Origlia N. Exogenous 3-Iodothyronamine Rescues the Entorhinal Cortex from β-Amyloid Toxicity. Thyroid. 2020 Jan;30(1):147-160. doi: 10.1089/thy.2019.0255.
Rutigliano G, Stazi M, Arancio O, Watterson DM, Origlia N. An isoform-selective p38α mitogen-activated protein kinase inhibitor rescues early entorhinal cortex dysfunctions in a mouse model of Alzheimer's disease. Neurobiol Aging. 2018 Oct;70:86-91. doi: 10.1016/j.neurobiolaging.2018.06.006.
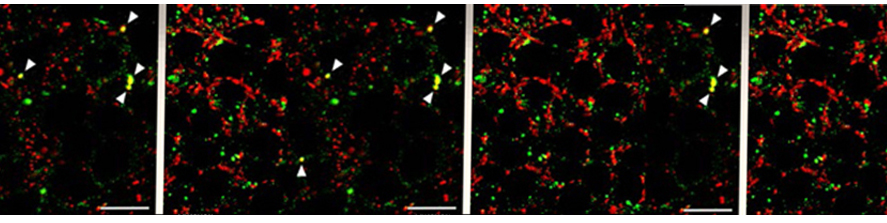
Criscuolo C, Fontebasso V, Middei S, Stazi M, Ammassari-Teule M, Yan SS, Origlia N. Entorhinal Cortex dysfunction can be rescued by inhibition of microglial RAGE in an Alzheimer's disease mouse model. Sci Rep. 2017 Feb 13;7:42370. doi: 10.1038/srep42370.
Origlia N, Criscuolo C, Arancio O, Yan SS, Domenici L. RAGE inhibition in microglia prevents ischemia-dependent synaptic dysfunction in an amyloid-enriched environment. J Neurosci. 2014 Jun 25;34(26):8749-60. doi: 10.1523/JNEUROSCI.0141-14.2014.