Colombo Sara
 Research scientist
Research scientist
c/o Università di Milano - Bicocca
Via Raoul Follereau, 3
20854 Vedano al Lambro
(MB)
Questo indirizzo email è protetto dagli spambots. È necessario abilitare JavaScript per vederlo.
tel. 02 64488374
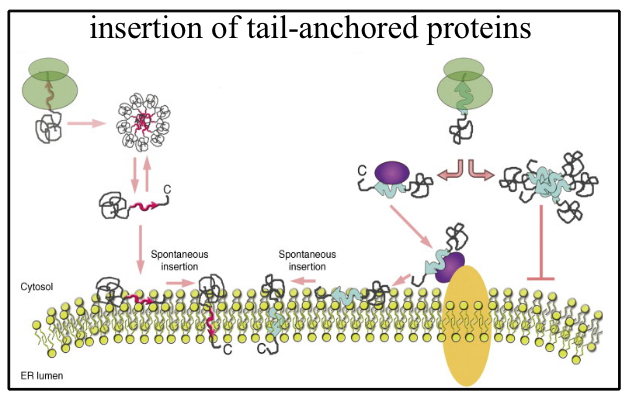
Mechanisms of insertion of membrane proteins into the ER
Research summary
Most membrane proteins are co-translationally inserted into the Endoplasmic Reticulum (ER) via the extensively investigated Sec61 protein-conducting channel. An exception to this rule is the insertion of so-called tail-anchored (TA) proteins. Because of the location of their transmembrane domain, their insertion into the phospolipid bilayer is necessarily post-translational. To investigate the mechanism of insertion of TA proteins into the ER membrane, we have developed stringent assays, based on protection from proteolysis of the C-terminal residues after translocation across the bilayer and on glycosylation of a consensus site engineered at the C-terminus of TA substrates. In attempting to identify the molecules involved in the insertion, we have now defined two classes of TA proteins: those with only moderately hydrophobic transmembrane domain (TMD) (type I TA proteins) are capable of translocation across protein-free bilayers without assistance from any membrane or cytosolic protein; those with more strongly hydrophobic TMDs, (type II) require a novel chaperone system (the GET system) which is presently being investigated by a number of laboratories. We are also attempting to define the molecular requirements for insertion of class II substrates in mammalian cells.
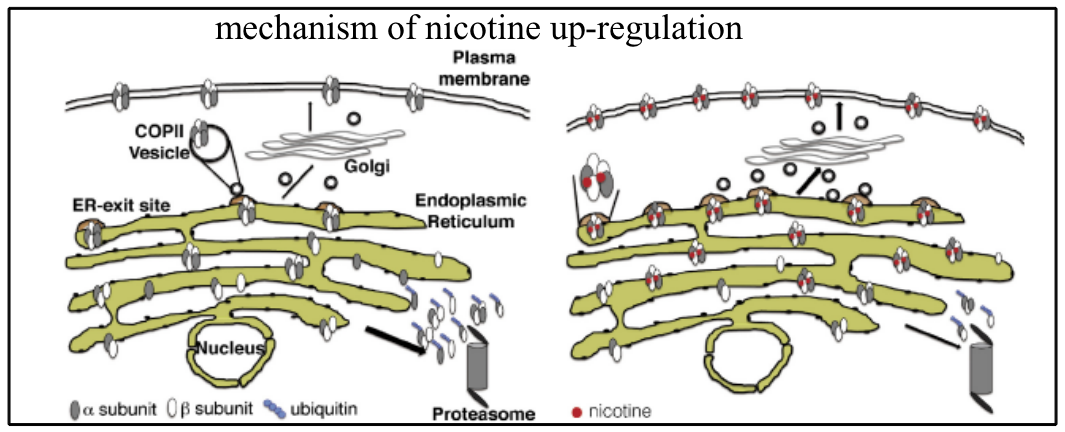
Mechanism of nicotine up-regulation of nAChRs
nAChRs (neuronal nicotinic acetylcholine receptors) are an heterogeneous family of oligomeric ACh -gated cation channels present in the central and peripheral nervous systems, which are formed by the pentameric assembly of identical α subunits (homomeric) or combinations of α and â subunits (heteromeric). The heteromeric receptor is composed of 2 α and 2 βsubunits, which generate 2 binding sites for Ach and nicotine at the two α/β subunit interfaces, and a fifth accessory subunit (α or β). The chronic treatment with nicotine up-regulates many nAChRs by a still unidentified mechanism. Recent human genetic studies have revealed a link between α3 and β4 subunits, whose genes are clustered with that of the accessory subunit α5, nicotine dependence phenotypes and lung cancer. In particular it was found that several single-nucleotide polymorphisms (SNPs) are associated with smoking-related behaviours. One of these SNPs leads to an asparagine-to-aspartic acid substitution in the nicotinic receptor α5 subunit at the amino acid position 398. We demonstrated that nicotine present during receptor synthesis favours assembly of the (α3)2(β4)3 pentamer, decreasing the proportion of the (α3)3(β4)2 receptors. The resulting receptor is less prone to proteasome degradation and can more easily exit the ER probably because of the presence of a higher number (3 instead of 2) of export motifs (LFM) that allow a more efficient recruitment of α3β4 to ER exit sites. In our reconstituted system we are currently investigating the role of the α5 subunit or of the α5 bearing the SNP398 in the expression and trafficking of the α3β4 subtype.
Representative publications
Mazzo F, Pistillo F, Grazioso G, Clementi F, Borgese N, Gotti C, Colombo SF. (2013). Nicotine-modulated subunit stoichiometry affects stability and trafficking of α3β4 nicotinic receptor, J Neurosci. 33(30): 12316-12328
Colombo SF, Mazzo F, Pistillo F, Gotti C. (2013). Biogenesis, trafficking and up-regulation of nicotinic ACh receptors, Biochem Pharmacol. 86(8):1063-73
Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P. (2013). PI(4,5)P2-Dependent and Ca(2+)-Regulated ER-PM Interactions Mediated by the Extended Synaptotagmins, Cell 153(7): 1494-1509
Grüring C, Heiber A, Kruse F, Flemming S, Franci G, Colombo SF, Fasana E, Schoeler H, Borgese N, Stunnenberg HG, Przyborski JM, Gilberger TW, Spielmann T. (2012). Uncovering common principles in protein export of malaria parasites, Cell Host Microbe 12(5): 717-729
Colombo S, Fasana E (2011). Mechanism of insertion of tail-anchored proteins into the membrane of the endoplasmic reticulum, Curr protein Pept Sci 12:736-742
Colombo S, Longhi R, Borgese N (2009). The role of cytosolic proteins in the insertion of tail anchored proteins into phospholipid bilayers, J Cell Sci 122:2383-2392
Ronchi P, Colombo S, Francolini M, Borgese N (2008). Transmembrane domain- dependent partitioning of membrane proteins within the endoplasmic reticulum, J Cell Biol 181: 105-118