Carmignoto Piergiorgio
 Research associate
Research associate
c/o Complesso Biologico Interdipartimentale
A. Vallisneri
Viale Giuseppe Colombo 3
35121 Padova
Tel 049-8276075
Fax 049-8276040
This email address is being protected from spambots. You need JavaScript enabled to view it.
Research summary
Reciprocal interactions between astrocytes and neurons in brain physiology and pathology.
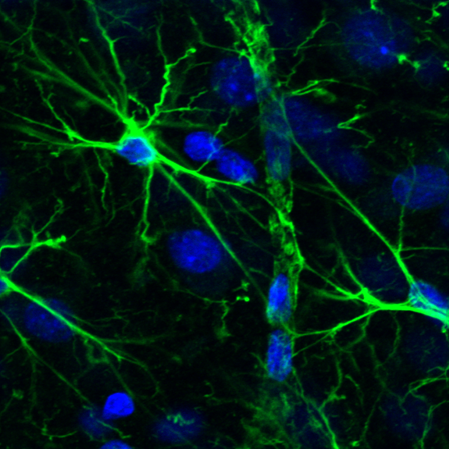
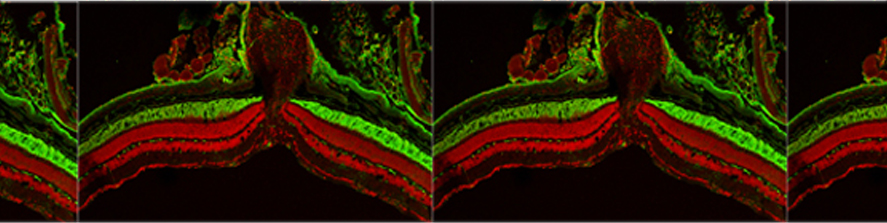
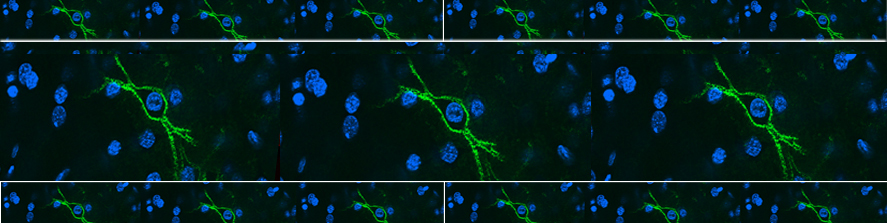
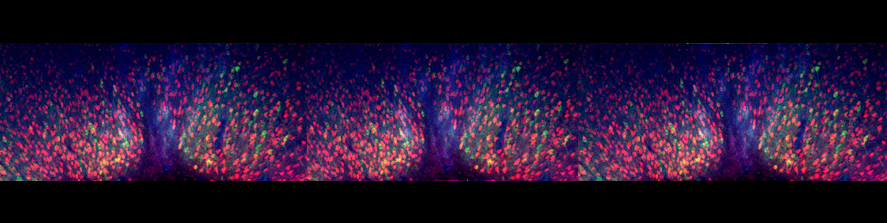
Astrocytic glial cells are key players in the control of brain tissue homeostasis. Recent works have extended the role of astrocytes also to the fine modulation of synaptic transmission and information processing in the brain, once considered to be exclusively neuronal functions. By releasing gliotransmitters (glutamate, ATP, D-serine, cytokines) astrocytes can modulate synapses with different mechanisms both at pre and postsynaptic level. Our main goal is to clarify how astrocytes modulate neuronal network activity in physiological and pathological conditions. Astrocytes involvement in very early steps of different neurological disorders like Alzheimer’s disease, Parkinson’s disease and epilepsy is supported by growing evidence, opening new unexploited therapeutic strategies. Our recent results show the involvement of astrocytes at seizure generation, pointing them as a novel target to treat seizures. Right panel: confocal fluorescence image of astrocytes (green) in slice of temporal cortex.
Main Research Results
1. Astrocytes favor seneration.
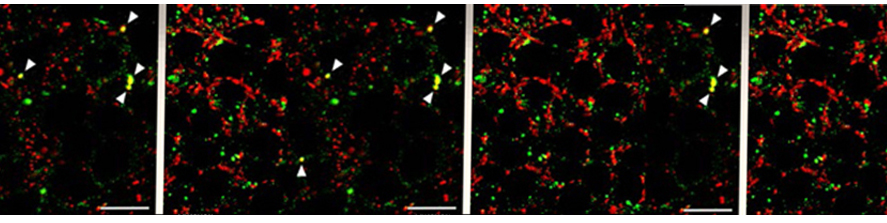
In brain slices we developed an experimental model that, by using local neuronal stimulation, has the unique advantage to evoke focal seizure like discharges from a known restricted site and at known timing (Losi et al 2010). New anticonvulsant molecules can be easily studied with this experimental approach as reproducible seizure like events can be repeatedly induced. This model makes also possible to study the early cellular events that take place in the area of ictal discharge generation (ictogenesis). Doing this we found that astrocytes are activated by neurons before the generation of seizure like events (Figure 1). Using different experimental approaches we revealed that astrocytic activation or inhibition in the focal area favors or impairs, respectively, the generation of seizure like events (Gomez-Gonzalo et al 2010). These data reveal the importance of astrocytes in ictogenesis and support the idea of targeting these glial cells to develop novel antiepileptic therapies.
2. Fast spiking GABAergic interneurons shape focal seizure propagation.
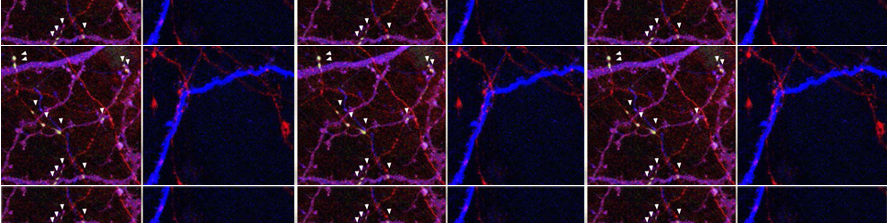
Parvalbumin fast-spiking (Pv-FS) interneurons represent the most abundant subclass of GABAergic cells. These cells axons contact soma and proximal dendrites of principal cells to efficiently modulate their firing activity. Recent results of our group revealed that the inhibitory activity that opposes, and occasionally prevents, ictal discharge propagation in temporal and entorhinal cortices are mainly due to Pv-FS interneurons activity (Cammarota et al 2013). Our data also show that seizure like discharge propagation occurs in modular groups of principal neurons that coincides with a sudden impairment of local Pv-FS interneurons firing activity that leads to a dramatic inhibitory failure (Cammarota et al 2013).
Ongoing Studies
We are characterizing the reciprocal interaction between GABAergic interneurons and astrocytes. In our laboratory we are using optogenetic tools like Channelrhodopsin-2 to selectively activate specific neuronal subclasses, like those expressing Pv or Som, to study their contribution to astrocytic activation and to epileptiform activity. The modulation of synaptic transmission by astrocytic activity is also under investigation in different experimental conditions.
Techniques used: intracellular calcium imaging with fast laser scanning microscopy (single or two-photon excitation) and simultaneous patch-clamp or field-potential recordings on brain slices and in vivo.
Representative publications
Crunelli V., Carmignoto G. and Steinhauser C. (2014). Novel astrocytic targets provide new avenues for therapeutic treatment of epilepsy. The Neuroscientists DOI: 10.1177/1073858414523320
Araque, A, Carmignoto G., Haydon P.G., Oliet S.H.R., Robitaille R., Volterra A. (2014). Gliotransmitters Travel in Time and Space. Neuron 81, 728-239.
Crunelli V, Carmignoto G. (2013). New vistas on astroglia in convulsive and non-convulsive epilepsy highlight novel astrocytic target for treatment. The Journal of Physiology 591, 775-785.
Cammarota M., Losi G., Chiavegato A., Zonta M. and Carmignoto G. (2013). Fast spiking interneuron control of seizure propagation in a cortical slice model of focal epilepsy. The Journal of Physiology 591, 807-822.
Gómez-Gonzalo M., Losi G, Chiavegato A. , Zonta M., Cammarota M., Brondi M., Vetri F., Uva L., Pozzan T., de Curtis M., Ratto G.M., Carmignoto G. (2010). An Excitatory Loop with Astrocytes Contributes to Drive Neurons to Seizure Threshold. PLoS Biology 8, 4, Doi:10.1371/journal.pbio.1000352.
Bardoni R., Ghirri A., Zonta M., Betelli C., Vitale Giovanni, Ruggieri Valentina V., Sandrini M. and Carmignoto G. (2010). Glutamate-mediated astrocyte-to-neuron signaling in the rat dorsal horn. The Journal of Physiology 588, 831-846.
Carmignoto G. and Gómez-Gonzalo M. (2010). The contribution of astrocyte signalling to neurovascular coupling. Brain Research Reviews 63, 138-148.
Fellin, T., Gomez-Gonzalo, M., Gobbo, S., Carmignoto, G. and Haydon P.G. (2006). Astrocytic glutamate is not necessary for the generation of epileptiform neuronal activity in hippocampal slices. The Journal of Neuroscience 26:9312-9322.
Haydon P.G. and Carmignoto G. (2006). Astrocyte control of synaptic transmission and neurovascular coupling. Physiological Reviews 86, 1009-1031.
Crippa D. , Schenk U., Francolini M., Rosa R., Verderio C., Zonta M., Pozzan T., Matteoli M., Carmignoto G. (2006). Synaptobrevin2-expressing vesicles in astrocytes: insights into molecular characterization, dynamics and exocytosis. The Journal of Physiology 570, 567-582.
Fellin T., Pascual O., Gobbo S., Pozzan T., Haydon P.G. and Carmignoto G. (2004). Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43, 729-743.
Fellin T. and Carmignoto G. (2004). Neuron-to-astrocyte signaling in the brain represents a distinct multifunctional unit. The Journal of Physiology 559, 3-15.
Zonta M., Sebelin A., Gobbo S., Fellin T., Pozzan T. and Carmignoto G. (2003). Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes. The Journal of Physiology 553, 407-414.
Zonta M., Angulo M.C., Gobbo S., Rosengarten B., Hossmann K.-A., Pozzan T. and Carmignoto G. (2003). Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nature Neuroscience 6, 43-50.
Carmignoto G., Pasti L. and Pozzan T. (1998). On the Role of Voltage-Dependent Calcium Channels in Calcium Signalling of Astrocytes in situ. The Journal of Neuroscience 18, 4637-4645.
Pasti L, Volterra A, Pozzan T and Carmignoto G. (1997). Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. The Journal of Neuroscience 17, 7817-7830.
Carmignoto G., Pizzorusso T., Tia S. and Vicini S. (1997). Brain-derived neurotrophic factor and nerve growth factor potentiate excitatory synaptic transmission in the rat visual cortex. The Journal of Physiology 498.1, 153-164.
Pasti L., Pozzan T. and Carmignoto G. (1995). Long-lasting changes of calcium oscillations in astrocytes: a new form of glutamate-mediated plasticity. Journal of Biological Chemistry 270, 15203-15210.
Carmignoto G. and Vicini S. (1992). Activity-dependent decrease in NMDA responses during development of the visual cortex. Science 258, 1007-1011.
Aggarwal VS, Liao J, Bondarev A, Schimmang T, Lewandoski M, Locker J, Shanske A, Campione M, Morrow BE (2006). Dissection of Tbx1 and Fgf interactions in mouse models of 22q11DS suggests functional redundancy. Hum Mol Genet. 15, 3219-28.
 Research associate
Research associate