Pallafacchina Giorgia
 Research scientist
Research scientist
c/o Complesso Biologico Interdipartimentale
A. Vallisneri
Viale Giuseppe Colombo 3
35121 Padova
Tel 049-8276481
Fax 049-8276040
This email address is being protected from spambots. You need JavaScript enabled to view it.
Personal website
Link to Lab group website
Mitochondria in cell signaling
Research topics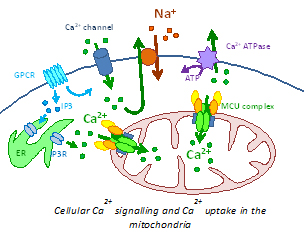
Since many years, the scientific interest of our lab is focused on the study of mitochondrial Ca2+homeostasis inside the cell which is widely recognized to be a fundamental signalling pathway in the intracellular communication. Indeed, rapid accumulation of Ca2+ within mitochondria depends on a steep driving force, provided by the electrochemical gradient generated by the respiratory chain, and by the establishment of close contact with the ER Ca2+ store. In addition, mitochondrial Ca2+ uptake, besides participating in buffering cytoplasmic Ca2+ increases, controls different processes within the cell: i) aerobic metabolism, through the stimulation of Ca2+-sensitive dehydrogenases of the matrix and substrate transporters ii) cell death, by favouring the opening of the permeability transition pore and hence release of caspase cofactors and iii) autophagic survival pathway, by exerting a suppressive role on one of the key molecules in autophagy induction, the AMPK kinase.
It is thus well known that mitochondrial Ca2+ uptake is a decisive step in the cellular signaling, but its study and its potential use for therapeutic purposes has been so far hampered by the lack of molecular information on the factor responsible for the entry of Ca2+ in the organelle.The situation was recently changed by the identification by our group of the mitochondrial Ca2+ channel (the “mitochondrial Ca2+ uniporter”, MCU, see De Stefani et al., 2011 Nature) which opened the route to the molecular (genetic and pharmacological) intervention over the mitochondrial Ca2+ dynamics. 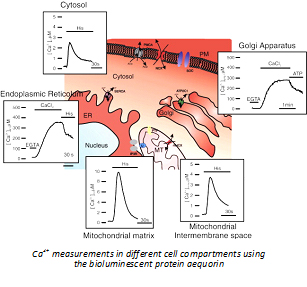 At present, our work combines the documented knowledge of the lab on the mitochondrial Ca2+ signaling with our established expertise on cellular models of human pathologies as well as on skeletal muscle physiology. Indeed, we study the molecular mechanisms and signaling pathways involved in the aetiopathology of different human diseases - from neurodegenerative to mitochondrial diseases - and in skeletal muscle hypertrophy and differentiation, with particular attention to the contribution of mitochondria and mitochondrial Ca2+ to these processes.
At present, our work combines the documented knowledge of the lab on the mitochondrial Ca2+ signaling with our established expertise on cellular models of human pathologies as well as on skeletal muscle physiology. Indeed, we study the molecular mechanisms and signaling pathways involved in the aetiopathology of different human diseases - from neurodegenerative to mitochondrial diseases - and in skeletal muscle hypertrophy and differentiation, with particular attention to the contribution of mitochondria and mitochondrial Ca2+ to these processes.
Ongoing projects
The main focus of our work is to define the mitochondrial Ca2+ signature in a variety of physiological and pathological conditions to assess the role of the organelle Ca2+ homeostasis in regulating cell responses.
Our present projects aim to:
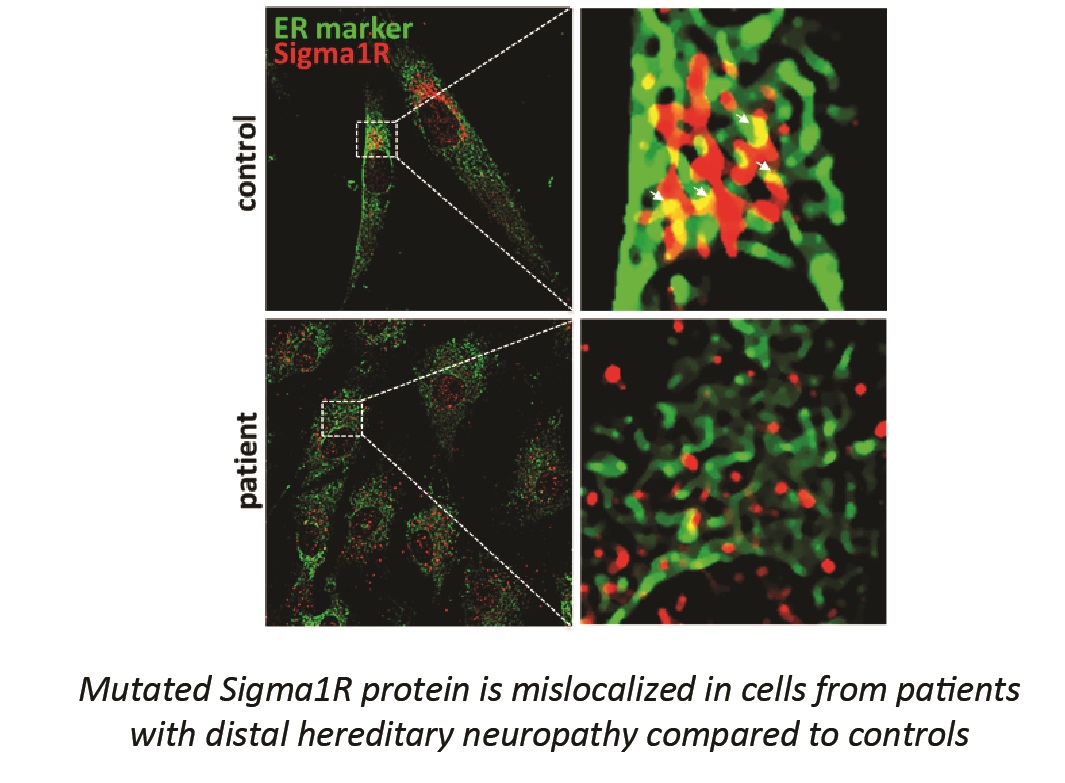
- study the involvement of mitochondrial Ca2+ in cellular models of human distal neuropathy (also known as spinal muscular atrophy) caused by mutation in Sigma1R protein consisting of both established cell lines and primary fibroblasts from affected patients;
- explore the role of mitochondrial Ca2+ during vertebrate development in the animal model of zebrafish (Danio rerio) by manipulating component of the MCU channel expression ;
 - define the contribution of the mitochondrial Ca2+signaling to the control of stem cell fate, in particular we are studying the mitochondrial Ca2+ signature during muscle-derived stem cell in vitro differentiation and its role in the regulation of satellite cells in vivo in adult muscle;
- define the contribution of the mitochondrial Ca2+signaling to the control of stem cell fate, in particular we are studying the mitochondrial Ca2+ signature during muscle-derived stem cell in vitro differentiation and its role in the regulation of satellite cells in vivo in adult muscle;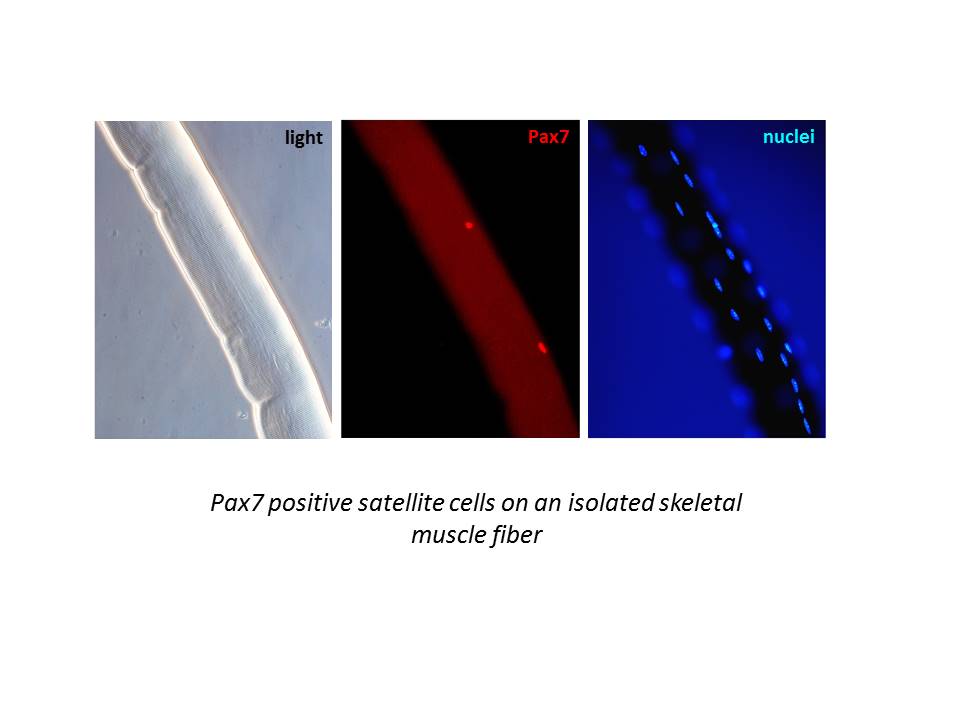
- identify the molecular origin of the mitochondrial dysfunction at the basis of the pathogenesis of Collagen VI muscular dystrophies, with particular interest to the Ca2+ deregulation and defective organelle clearance and their consequences on energy metabolism of muscle cells.
Representative publications
Gregianin E*, Pallafacchina G*, Zanin S, Crippa V, Rusmini P, Poletti A, Fang M, Li Z, Diano L, Petrucci A, Lispi L, Cavallaro T, Fabrizi GM, Muglia M, Boaretto F, Vettori A, Rizzuto R, Mostacciuolo ML, Vazza G. Loss-of-function mutations in the SIGMAR1 gene cause distal hereditary motor neuropathy by impairing ER-mitochondria tethering and Ca2+ signaling. Hum Mol Genetics. 2016 Sept 1; 25(17):3741-53. (* co-first authors).
Granatiero V, Giorgio V, Calì T, Patron M, Brini M, Bernardi P, Tiranti V, Zeviani M, Pallafacchina G*, De Stefani D*, Rizzuto R*. Reduced mitochondrial Ca2+ transients stimulate autophagy in human fibroblasts carrying the 13514A>G mutation of the ND5 subunit of NADH dehydrogenase. Cell Death Differ. 2016 Feb;23(2):231-41. (*co-corresponding authors).
Mammucari C, Gherardi G, Zamparo I, Raffaello A, Boncompagni S, Chemello F, Cagnin S, Braga A, Zanin S, Pallafacchina G, Zentilin L, Sandri M, De Stefani D, Protasi F, Lanfranchi G, Rizzuto R. The mitochondrial calcium uniporter controls skeletal muscle trophism in vivo. Cell Rep. 2015 Mar 3;10(8):1269-79.
Patron M, Raffaello A, Granatiero V, Tosatto A, Merli G, De Stefani D, Wright L, Pallafacchina G, Terrin A, Mammucari C, Rizzuto R. The mitochondrial calcium uniporter (MCU): molecular identity and physiological roles. J Biol Chem. 2013 Apr 12;288(15):10750-8.
Pallafacchina, G., Blaauw, B., and Schiaffino, S. Role of satellite cells in muscle growth and maintenance of muscle mass. Nutr Metab Cardiovasc Dis 2013 Dec; 23 Suppl 1, S12-18.
Pallafacchina, G., Francois, S., Regnault, B., Czarny, B., Dive, V., Cumano, A., Montarras, D., and Buckingham, M. An adult tissue-specific stem cell in its niche: a gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem Cell Res 2010; 4, 77-91.
Pallafacchina G., Calabria E., Serrano A.L., Kalhovde J.M. and Schiaffino S.. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci U S A 2002, 99:9213-18.