Lab chemistry and biology of natural compounds
Chemistry and biology of natural compounds

See also Mario Zoratti’s and Lucia Biasutto’s personal pages
Developing a pharmacology of polyphenols
Background
Polyphenols are a vast family of natural compounds exhibiting, at least in vitro, a variety of activities of potential relevance for such major health-care endeavours as protection of the cardiovascular system, improving intellectual performance impaired by old age or neurodegeneration, and prevention and therapy of cancer. The notoriously low bioavailability of these compounds is a major obstacle for their pharmacological exploitation. Since they are ready-made Phase II metabolism substrates, they are rapidly converted by sulfo- and glucuronyl-transferases in enterocytes into conjugates which are to a large extent re-exported to the intestinal lumen. Liver enzymes then intervene on the molecules which have entered the circulation, administering other rounds of Phase II metabolism.
Project
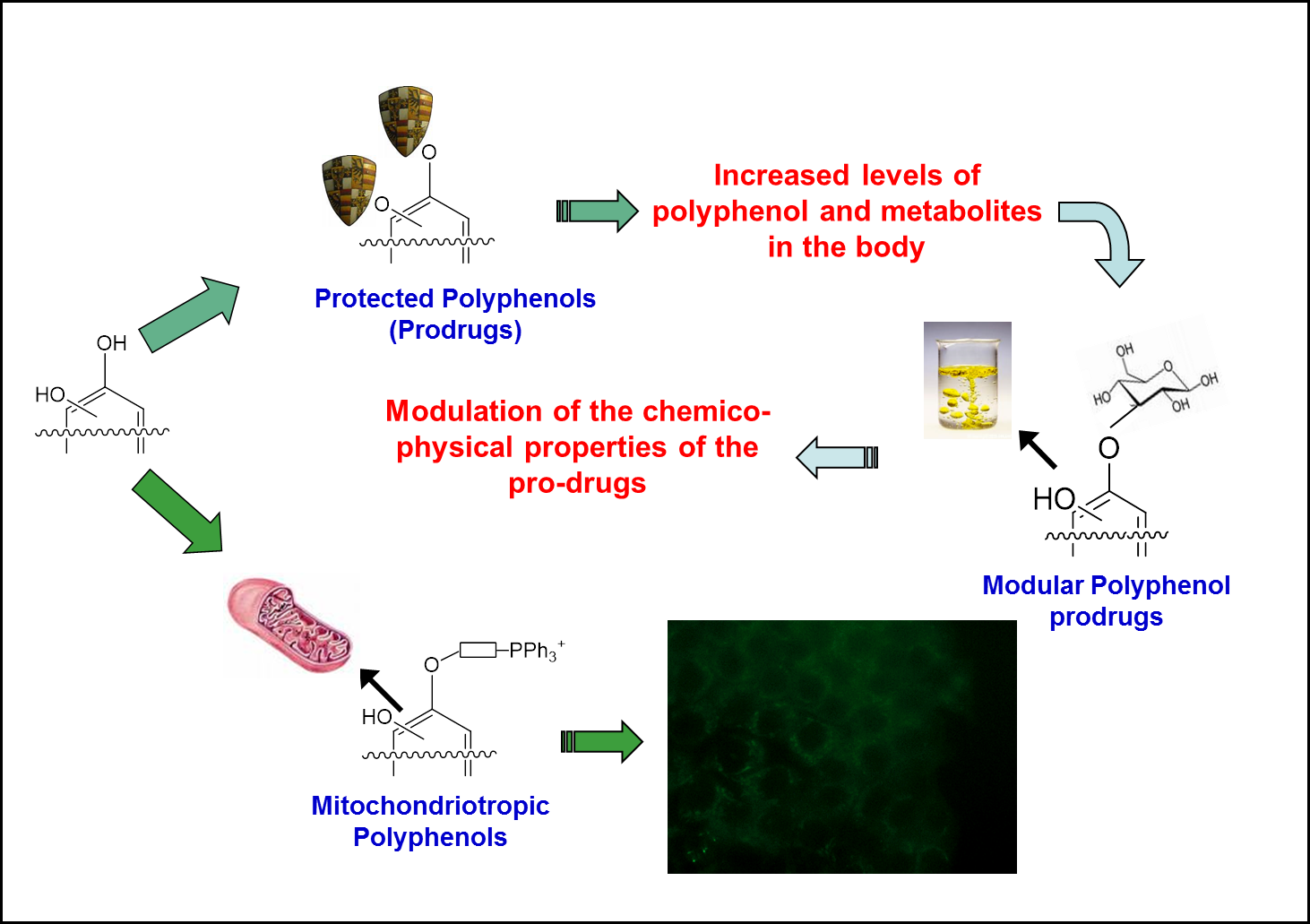
The goal of the program is to find ways to increase the concentration of polyphenols and their conjugates in the body or at specific locations in the body, considering them as potential pharmacological agents. Three related approaches, all based on the chemical synthesis of novel derivatives, are actively pursued:
• Prodrugs. Precursors are devised in which the polyphenol hydroxyls, the sites of conjugation, are protected during absorption, regenerating the parent compound through enzymatic action after the crucial initial period.
• Mitochondriotropic derivatives. In this case a mitochondria-targeting permeant cation is attached to the polyphenol, determining accumulation in the matrix of mitochondria, a site of intense redox activity.
• Solubilisation. A recurrent problem is the low solubility in water of polyphenol “aglycones” and their derivatives. We are therefore developing precursors incorporating solubilising groups and maintaining the ability to permeate biomembranes.
Collaboration
The project is a joint enterprise with Prof. Cristina Paradisi’s group, which carries out all chemical synthesis and characterization work at the Dept. of Chemical Sciences of the University of Padova.

5 recent publications
Amino Acid Carbamates As Prodrugs Of Resveratrol.
Mattarei A, Azzolini M, La Spina M, Zoratti M, Paradisi C, Biasutto L.
Sci Rep. 2015 Oct 14;5:15216.
Pharmacokinetics and tissue distribution of pterostilbene in the rat.
Azzolini M, La Spina M, Mattarei A, Paradisi C, Zoratti M, Biasutto L.
Mol Nutr Food Res. 2014 Nov;58(11):2122-32.
Cytotoxicity of mitochondria-targeted resveratrol derivatives: interactions with respiratory chain complexes and ATP synthase.
Sassi N, Mattarei A, Azzolini M, Szabo' I, Paradisi C, Zoratti M, Biasutto L.
Biochim Biophys Acta. 2014 Oct;1837(10):1781-9.
Prodrugs of quercetin and resveratrol: a strategy under development.
Biasutto L, Zoratti M.
Curr Drug Metab. 2014 Jan;15(1):77-95.
Acetal derivatives as prodrugs of resveratrol.
Mattarei A, Azzolini M, Carraro M, Sassi N, Zoratti M, Paradisi C, Biasutto L.
Mol Pharm. 2013 Jul 1;10(7):2781-92.
Mechanisms of action of polyphenols
We have recently begun a program intended to clarify mechanistic aspects of the bioactivity of selected polyphenols (pterostilbene and resveratrol). We are focusing on autophagy induction and on the impact of pterostilbene, in particular, on signalling and gene expression in the brain of rodents. This latter line of research is pursued in collaboration with the groups led by Alessandro Sale and Nicoletta Berardi at the CNR Institute of Neuroscience – Pisa.
Mitochondrial ion channels
We collaborate with the group led by Prof. Ildikò Szabò at the Dept. of Biology, University of Padova, and others, to identify and characterise ion-conducting channels of the mitochondrial inner membrane (IMM). We study their roles in mitochondrial and cellular physiology, and their possible exploitation as targets of pharmacological intervention. We focus in particular on the Permeability Transition Pore and on the IMM population of K+-selective Kv1.3 channels.
5 recent publications
Mattarei A, Azzolini M, La Spina M, Zoratti M, Paradisi C, Biasutto L.
Amino Acid Carbamates As Prodrugs Of Resveratrol. Sci Rep. 2015 Oct 14;5:15216.
Azzolini M, La Spina M, Mattarei A, Paradisi C, Zoratti M, Biasutto L.
Pharmacokinetics and tissue distribution of pterostilbene in the rat., Mol Nutr Food Res. 2014 Nov;58(11):2122-32.
Sassi N, Mattarei A, Azzolini M, Szabo' I, Paradisi C, Zoratti M, Biasutto L.
Cytotoxicity of mitochondria-targeted resveratrol derivatives: interactions with respiratory chain complexes and ATP synthase, Biochim Biophys Acta. 2014 Oct;1837(10):1781-9.
Biasutto L, Zoratti M.
Prodrugs of quercetin and resveratrol: a strategy under development, Curr Drug Metab. 2014 Jan;15(1):77-95.
Mattarei A, Azzolini M, Carraro M, Sassi N, Zoratti M, Paradisi C, Biasutto L.
Acetal derivatives as prodrugs of resveratrol, Mol Pharm. 2013 Jul 1;10(7):2781-92.
Supported By:
 Fondazione Cassa di Risparmio di Padova e Rovigo
Fondazione Cassa di Risparmio di Padova e Rovigo