Zonta Micaela
- Dettagli
- Visite: 6557
 Senior Technologist
Senior Technologist
c/o Complesso Biologico Interdipartimentale
A. Vallisneri
Viale Giuseppe Colombo 3
35121 Padova
Tel 0049-8276359
Questo indirizzo email è protetto dagli spambots. È necessario abilitare JavaScript per vederlo.
Neuron-glia signaling in brain function and dysfunction
(Astrolábos)
Research summary
|
|
|
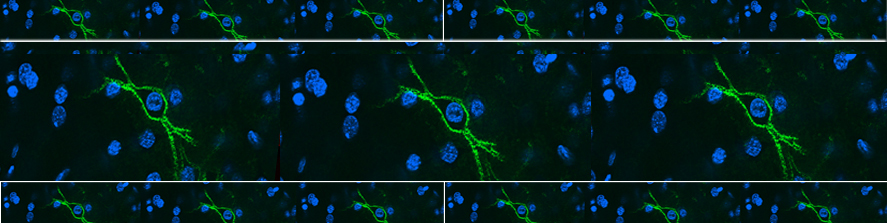
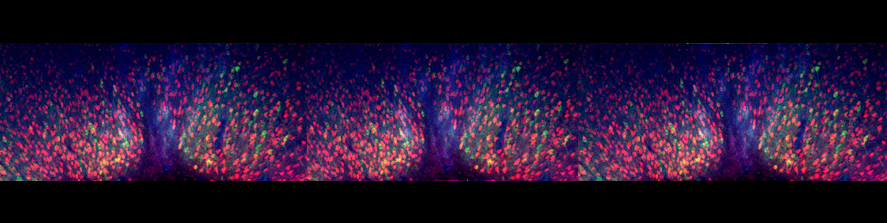
Astrocytes are glial cells playing key roles in the control of brain tissue homeostasis.
A growing body of experimental evidence indicates that astrocytes also contribute to the fine modulation of synaptic transmission and information processing in the brain - processes once considered exclusive to neurons. Moreover, these cells are implicated in several neurological disorders, including Alzheimer’s disease, Parkinson’s disease and epilepsy, thereby opening novel opportunities for the development of previously unexplored therapeutic strategies. The main goal of our research is to investigate neuron-astrocyte reciprocal communication, with the final aim to disclose how astrocytes modulate neuronal network activity and overall brain function in physiological and pathological conditions. A specific focus of the group is the study of Ca2+ signal in astrocytes, coupled to electrophysiological recordings in acute brain slices and behavioural assessment.
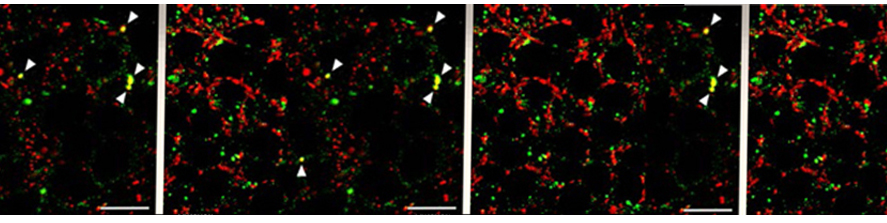
Our group, previously led by Dr. Giorgio Carmignoto, has contributed to the current knowledge in the field by describing, for instance, the regulation of gliotransmitter release by Ca2+ dynamics, the involvement of astrocytes in neurovascular coupling, their recruitment by neuronal activity and their contribution to epileptic activity.
More recently, we revealed the contribution of astrocytes to the synaptic modulation of the dopaminergic circuits of Ventral Tegmental Area (Requie et al., 2022) and characterized the Ca2+ response evoked in VTA astrocytes by the neuromodulator norepinephrine (Speggiorin et al., 2024).
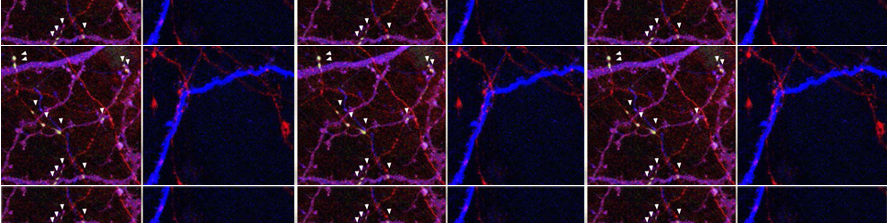
We also demonstrated the negative effects of early astrocyte Ca2+ dysregulation on the synaptic plasticity of somatosensory circuits in a mouse model of Alzheimer’s disease (B6.152H) and developed an astrocyte-based rescue strategy (Lia et al., 2023). In parallel, we are completing the characterization of astrocyte Ca2+ signal in another mouse model displaying Alzheimer’s disease like phenotype.
|
|
 |
Ongoing projects
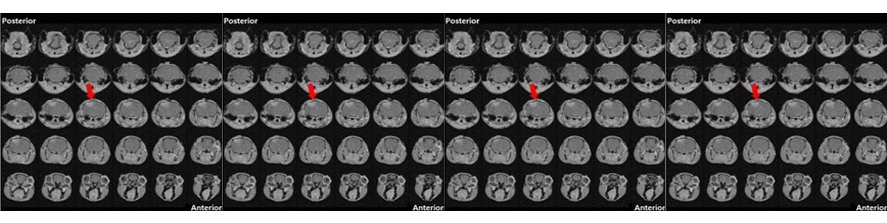
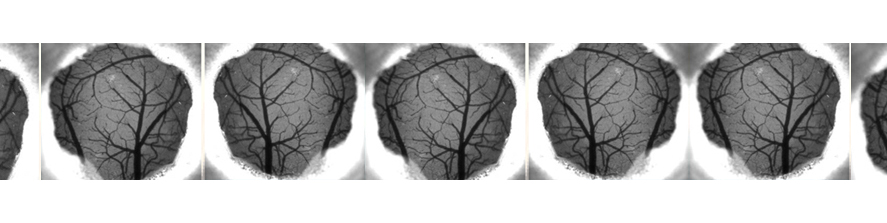
In a recently funded Telethon project, the lab is exploring cerebrovascular function in the B6.152H model, to disclose possible links between the impairment in astrocyte Ca2+ signal and the different aspects of vascular dysfunction commonly reported in Alzheimer’s disease.
In parallel, we are studying the contribution of VTA astrocytes to the behaviour driven by stress conditions. Recently, we also started investigating the role of astrocytes in neuro-oncological mechanisms.
Staff
Micaela Zonta, CNR Senior Technologist
Marta Gómez-Gonzalo, CNR Senior Researcher
Angela Chiavegato, UniPD Technician and CNR Associate
Alessandro Di Spiezio, Telethon Post-doc
Nicole Tonesi, PhD student
Federico Dal Ferro, Master thesis student
Giorgio Carmignoto, CNR Senior Associate
Selected publications
- Di Spiezio A, Gomez-Gonzalo M, Chiavegato A and Zonta M. Recent Insights on Supraspinal Astrocytes in Chronic Pain. Neurochemical Research 2025.
- Gómez-Gonzalo M. Astrocytes in Rodent Anxiety-Related Behavior: Role of Calcium and Beyond. International Journal of Molecular Sciences 2025.
- Speggiorin M, Chiavegato A, Zonta M, Gómez-Gonzalo M. Characterization of the Astrocyte Calcium Response to Norepinephrine in the Ventral Tegmental Area. Cells 2024.
- Lia A, Sansevero G, Chiavegato A, Sbrissa M, Pendin D, Mariotti L, Pozzan T, Berardi N, Carmignoto G, Fasolato C and Zonta M. Rescue of astrocyte activity by the calcium sensor STIM1 restores long-term synaptic plasticity in female mice modelling Alzheimer’s disease. Nature Communications 2023.
- Lia A, Di Spiezio A, Speggiorin M and Zonta M. Two decades of astrocytes in neurovascular coupling. Frontiers in Network Physiology 2023.
- Requie L, Gómez-Gonzalo M, Speggiorin M, Managò F, Melone M, Congiu M, Chiavegato A, Lia A, Zonta M, Losi G, Henriques V. J, Pugliese A, Pacinelli G, Giovanni M, Papaleo F, Muntoni A.L, Conti F and Carmignoto G. Astrocytes mediate long-lasting synaptic regulation of Ventral Tegmental Area dopamine neurons. Nature Neuroscience 2022.