Matteoli Michela
- Dettagli
- Visite: 17016
Research associate URT c/o Humanitas Research Hospital
URT c/o Humanitas Research Hospital
Via Manzoni 56
20089 Rozzano
Cell biology of the synapse: deciphering the role of genetic and environmental factors in synapse function and dysfunction
Research summary
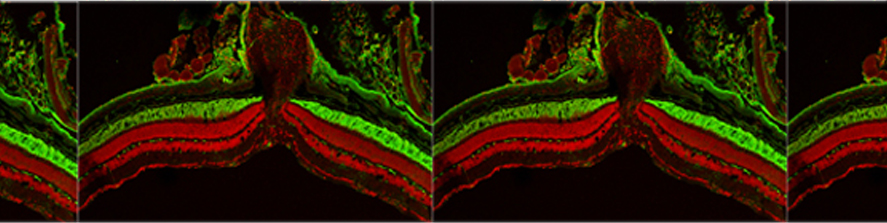
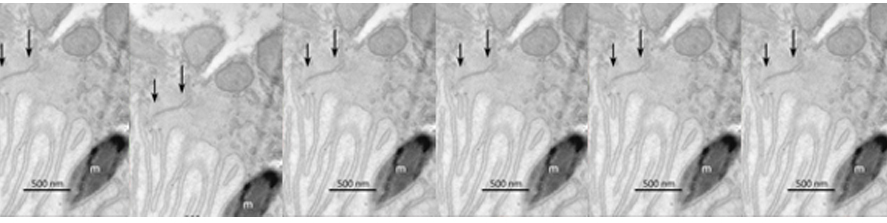
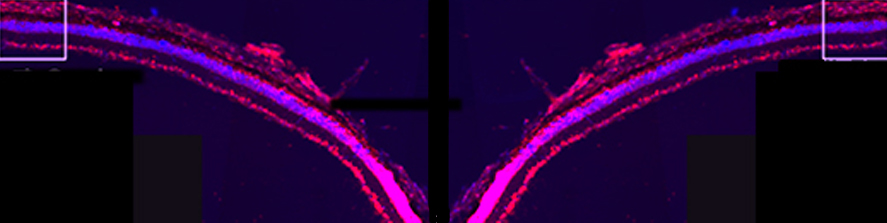
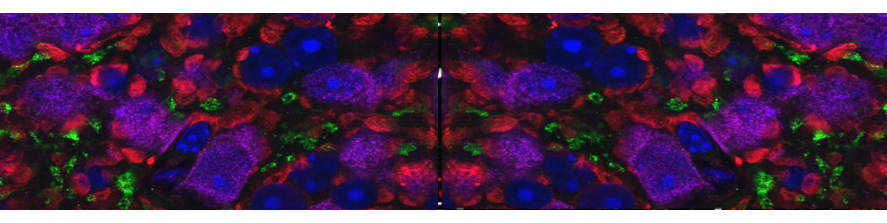
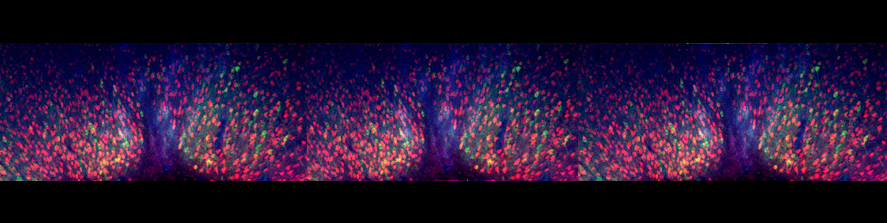
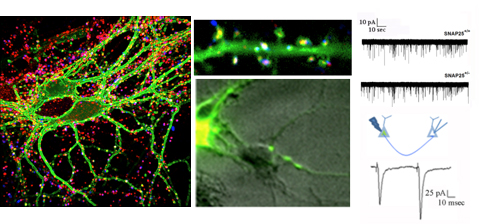
The main interest of the laboratory is the study of the mechanisms involved in the formation and function of the synapse -the central element that allows the communication between neurons in brain- and in defining how these processes get wrong in brain diseases. Synapses are fundamental brain structures that mediate information transfer between nerve cells. Signal transmission and information processing at synapses control all body functions and all aspects of cognition, including attention, perception, learning, decision making, as well as mood and affect. Since several years it has been recognized that many psychiatric and neurodegenerative disorders are synaptopathies, i.e. pathological conditions where the molecular and cellular pathways operating at the synapse are dysregulated. Recent contributions of the lab to the field of synaptopathies and neuroinflammation include the identification of the role of the SNARE protein SNAP-25 (involved in ADHD and schizophrenia) in the modulation of calcium channels and network excitability and the demonstration that reduced protein levels impact cognitive processes, altering short term plasticity and spine morphology.
More recently, evidence was provided that inflammation can speculatively modify the risk and/or severity of a variety of brain diseases. Although several molecules involved in inflammatory processes have been found to regulate specific neuronal processes, the processes by which inflammation cascades impact synapse formation and function, contributing to the onset of a brain disease is not defined. The research activity of the Laboratory aims at understanding the molecular mechanisms at the basis of synapse dysfunctions in neurodegenerative and psychiatric diseases and at defining whether key immune molecules impact this process, in order to identify new targets suitable for therapeutic interventions.
Representative publications
Corradini I, E Focchi E, Rasile M, Morini, R, Desiato G, Tomasoni R, Lizier M, Ghirardini E, Morone D, I Barajon I, Antonucci Fl, Pozzi D, Matteoli M, (2018), Maternal immune activation delays excitatory-to-inhibitory gaba switch in the offspring. Biol Psychiatry. 2017 Nov 14
Tomasoni R, Morini R, Lopez-Atalaya J P., Corradini I, Canzi A, Rasile M, Mantovani C, Pozzi D, Garlanda C, Mantovani A, Menna E, A Barco A, Matteoli M, (2017), Lack of IL-1R8in neurons causes hyperactivation of IL-1 receptor pathway and induces MeCP2-dependent synaptic defects. eLife Mar 28;6. pii: e21735
Mazzitelli S, Rasile M, Filipello F, Lauranzano E, Starvaggi CucuzzaC, Tamborini M, Pozzi D, Barajon I, Giorgino T, Natalello A, Matteoli M (2016), Beta amyloid 1-24 c-ter truncated fragment promotes beta amyloid 1-42 aggregate formation in the healthy brain, Acta Neuropathologica Communications 2016 4:110
Tamborini M, Locatelli E, Rasile M, Monaco I, Rodighiero S, Corradini I , Comes Franchini M, Passoni L, M Matteoli M (2016), A novel approach combining CTX-nanovectors and low dose radiation to reach infiltrating tumor niches in glioblastoma, ACS Nano. 2016 Feb 23;10(2):2509-20.
Fossati G, Morini R, Corradini I, Antonucci F, Trepte P, Edry E, Sherma V, Papale A, Pozzi D, Defilippi P, Meier J C., Brambilla R, Turco E, Rosenblum K, Wanker EE., Ziv N E., Menna E, Matteoli M, (2015), Reduced SNAP-25 Increases PSD-95 Mobility and Impairs Spine Morphogenesis, Cell Death and Differentiation Feb 13. doi: 10.1038/cdd.2014.227. [Epub ahead of print].
Antonucci F, Corradini I, Morini R, Fossati G, Menna E, Pozzi D, Pacioni S, Verderio C, Bacci A, Matteoli M. (2013), Reduced SNAP-25 alters short-term plasticity at developing glutamatergic synapses. (2013) EMBO Rep. 14(7):645-51. doi: 10.1038/embor.2013.75.
Tomasoni R, Repetto D, Morini R, Elia C, Gardoni F, Di Luca M, Turco E, Defilippi P, Matteoli M. (2013), SNAP-25 regulates spine formation through postsynaptic binding to p140Cap. Nat Commun. 4:2136. doi: 10.1038/ncomms3136.
Delamarche E, Tonna N, Lovchik R, Bianco F, Matteoli M, (2013), Pharmacology on microfluidics : Multimodal analysis for studying cell-cell interaction (2013). Curr Opinion Pharmacol.Oct;13(5):821-828. doi: 10.1016/j.coph.2013.07.005. Epub 2013 Jul 19
Corradini I, Donzelli A, Antonucci F, Welzl H, Loos M, Martucci R, De Astis S, Pattini L, Inverardi F, Wolfer D, Caleo M, Bozzi Y, Verderio C, Frassoni C, Braida D, Clerici M, Lipp HP, Sala M, Matteoli M, (2012) Epileptiform Activity and Cognitive Deficits in SNAP-25+/- Mice are Normalized by Antiepileptic Drugs. Cereb Cortex. [Epub ahead of print]
Condliffe SB, Corradini I, Pozzi D, Verderio C, Matteoli M. (2010), Endogenous SNAP-25 regulates native voltage-gated calcium channels in glutamatergic neurons. J Biol Chem. 285(32):24968-76. doi: 10.1074/jbc.M110.145813.
Pozzi D, Condliffe S, Bozzi Y, Chikhladze M, Grumelli C, Proux-Gillardeaux V, Takahashi M, Franceschetti S, Verderio C, Matteoli M. (2008), Activity-dependent phosphorylation of Ser187 is required for SNAP-25-negative modulation of neuronal voltage-gated calcium channels. Proc Natl Acad Sci U S A 105(1):323-8.
Verderio C, Pozzi D, Pravettoni E, Inverardi F, Schenk U, Coco S, Proux-Gillardeaux V, Galli T, Rossetto O, Frassoni C, Matteoli M. (2004), SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron 19;41(4):599-610.