Broccoli Vania
- Dettagli
- Visite: 15952
 Director of Research
Director of Research
Via Olgettina 58
20132 Milano
Tel 02-26434616
Fax 02-7490574
Questo indirizzo email è protetto dagli spambots. È necessario abilitare JavaScript per vederlo.
Neurological diseases affect profoundly society at every level causing a severe burden to patients and families.
Thought the last years have witnessed an unprecedented gain in knowledge on their pathophysiological bases, this has not yet led to a transformation of clinical interventions and their curative potential. In fact, neurological disorders pose additional great challenges hidden in the magnificent complexity of the cellular architecture and relative isolation of the brain tissue and the yet uncompleted understanding of its mechanisms of function.
To contribute to overcome these hurdles, we are developing new humanized models of neurological diseases employing the patient iPSC-derived human neural cells, organoids and microfluidic-organized neuronal circuitries. Moreover, we are working in improving gene-based therapies to correct gene mutations and restore correct gene expression in the brain to halt disease progression or even prevent the onset of the pathology.
To reach these goals, we are generating novel therapeutic viral capsids, optimizing administration routes and advancing CRISPR/Cas9 gene editing tools for safe and efficient gene repair in neural tissues. These activities are intimately associated to further progress our understanding of the neurological diseases and untangle the early molecular dysfunctions that contribute to pathological progression.
On this aspect, we are interested to comprehend the basis of vulnerability of certain neuronal populations to specific diseases and understand the different degrees of plasticity of neuronal cells during aging and cellular reprogramming.

Recent publications:
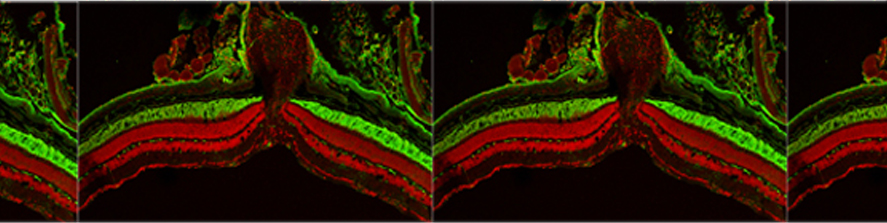
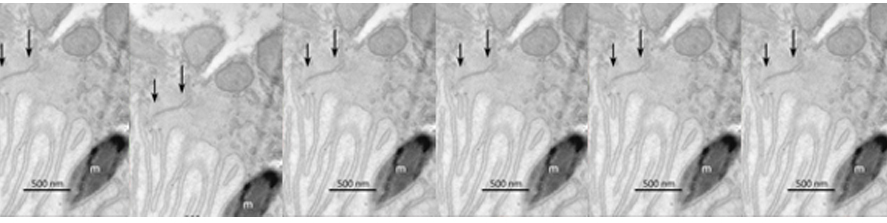
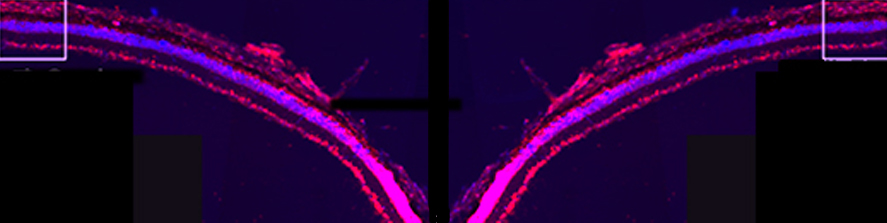
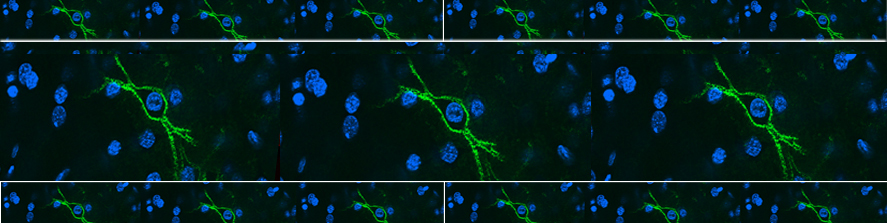
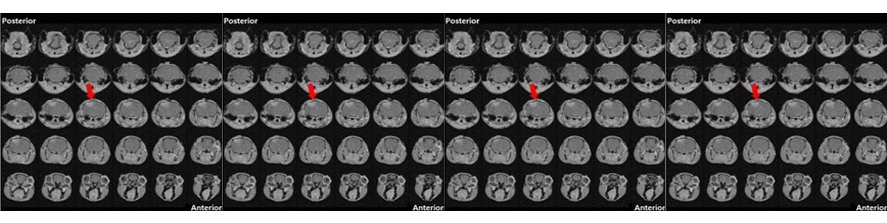
Rossi G, Ordazzo G, Vanni NN, Castoldi V, Iannielli A, Di Silvestre D, Bellini E, Bernardo L, Giannelli SG, Luoni M, Muggeo S, Leocani L, Mauri P, Broccoli V; MCT1-dependent energetic failure and neuroinflammation underlie optic nerve degeneration in Wolfram syndrome mice. Elife. 2023 Jan 16;12:e81779. doi: 10.7554/eLife.81779.
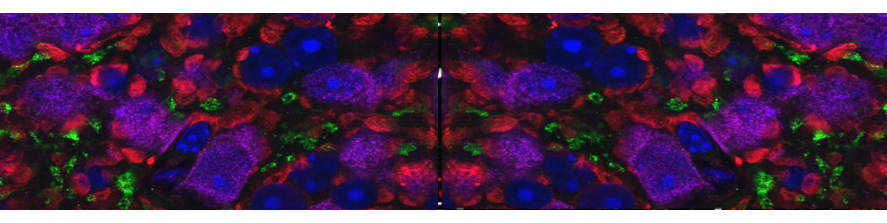
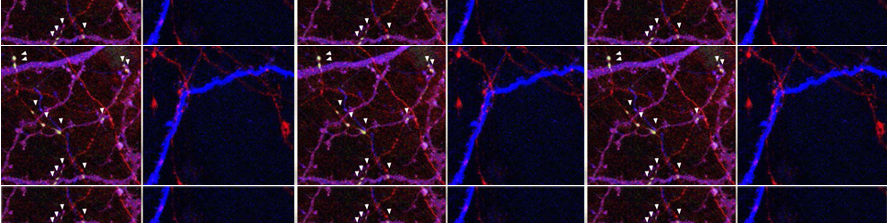
Iannielli A, Luoni M, Giannelli SG, Ferese R, Ordazzo G, Fossati M, Raimondi A, Opazo F, Corti O, Prehn JHM, Gambardella S, Melki R, Broccoli V.; Modeling native and seeded Synuclein aggregation and related cellular dysfunctions in dopaminergic neurons derived by a new set of isogenic iPSC lines with SNCA multiplications. Cell Death Dis. 2022 Oct 19;13(10):881. doi: 10.1038/s41419-022-05330-6.
Benedetti V, Banfi F, Zaghi M, Moll-Diaz R, Massimino L, Argelich L, Bellini E, Bido S, Muggeo S, Ordazzo G, Mastrototaro G, Moneta M, Sessa A, Broccoli V.; A SOX2-engineered epigenetic silencer factor represses the glioblastoma genetic program and restrains tumor development. Science Advance 2022 Aug 5;8(31):eabn3986. doi: 10.1126/sciadv.abn3986.
Valassina N, Brusco S, Salamone A, Serra L, Luoni M, Giannelli S, Bido S, Massimino L, Ungaro F, Mazzara PG, D'Adamo P, Lignani G, Broccoli V, Colasante G.; Scn1a gene reactivation after symptom onset rescues pathological phenotypes in a mouse model of Dravet syndrome. Nature Communications 2022 Jan 10;13(1):161. doi: 10.1038/s41467-021-27837-w.
Fumagalli V, Ravà M, Marotta D, Di Lucia P, Laura C, Sala E, Grillo M, Bono E, Giustini L, Perucchini C, Mainetti M, Sessa A, Garcia-Manteiga JM, Donnici L, Manganaro L, Delbue S, Broccoli V, De Francesco R, D'Adamo P, Kuka M, Guidotti LG, Iannacone M.; Administration of aerosolized SARS-CoV-2 to K18-hACE2 mice uncouples respiratory infection from fatal neuroinvasion. Science Immunology 2022 Jan 28;7(67):eabl9929. doi: 10.1126/sciimmunol.abl9929.
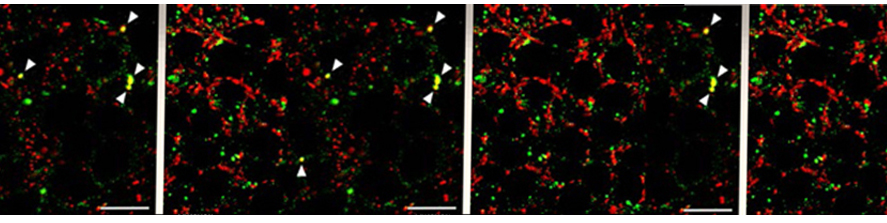
Bido S, Muggeo S, Massimino L, Marzi MJ, Giannelli SG, Melacini E, Nannoni M, Gambarè D, Bellini E, Ordazzo G, Rossi G, Maffezzini C, Iannelli A, Luoni M, Bacigaluppi M, Gregori S, Nicassio F, Broccoli V.; Microglia-specific overexpression of α-synuclein leads to severe dopaminergic neurodegeneration by phagocytic exhaustion and oxidative toxicity. Nature Communications 2021 Oct 29;12(1):6237. doi: 10.1038/s41467-021-26519-x.
Banfi F, Rubio A, Zaghi M, Massimino L, Fagnocchi G, Bellini E, Luoni M, Cancellieri C, Bagliani A, Di Resta C, Maffezzini C, Ianielli A, Ferrari M, Piazza R, Mologni L, Broccoli V, Sessa A.; SETBP1 accumulation induces P53 inhibition and genotoxic stress in neural progenitors underlying neurodegeneration in Schinzel-Giedion syndrome. Nature Communications 2021 Jun 30;12(1):4050. doi: 10.1038/s41467-021-24391-3.