Leonzino Marianna
- Details
- Hits: 2422
 Research scientist
Research scientist
URT c/o Humanitas Research Hospital
Via Manzoni 56
20089 Rozzano
Tel +39 02 82245251
Fax +39 02 82245290
This email address is being protected from spambots. You need JavaScript enabled to view it.
Scientific career path
Elucidating the molecular mechanism of cellular processes linked to neurological diseases has consistently been my main research interest. I did my PhD in the lab of Dr. Bice Chini (CNR - Milan, Italy), where I studied an oxytocin-dependent effect on the maturation of GABAergic signaling that has relevance for autism pathogenesis. Our findings revealed an oxytocin-mediated signaling pathway ensuring the proper developmental timing of the maturation of GABA-mediated neurotransmission. For my postdoctoral training in the De Camilli lab at Yale University (New Haven, CT- USA), I chose to focus on the role of intracellular lipid homeostasis in neurodegenerative diseases. My research, done in collaboration with the Reinisch lab (Dept. Cell Biology, Yale University), focused on the VPS13 protein family, whose 4 human genes are mutated in neurological diseases, including a Huntington-like syndrome, early onset Parkinson’s and a severe and heterogenous form of ataxia.
Recent scientific accomplishments
Distribution of membrane lipids from their production sites in the endoplasmic reticulum (ER) to all subcellular membranes relies on two mechanisms: vesicular transport and protein-mediated lipid transfer. While most subcellular compartments can rely on both mechanisms, mitochondria, that are excluded from vesicular membrane traffic, can only receive lipids via lipid transfer proteins. Lipid transfer proteins are preferentially localized at membrane contact sites, areas where membranes of different organelles are tethered in close proximity by proteins bridges, facilitating inter-organellar communication and nutrients exchange.
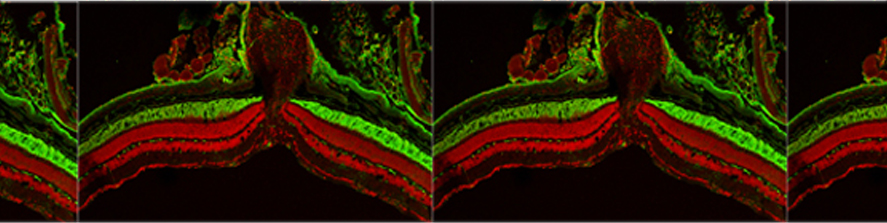
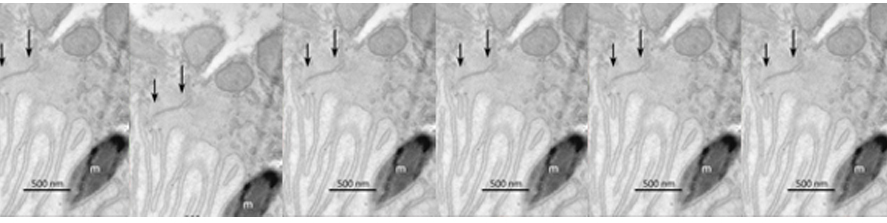
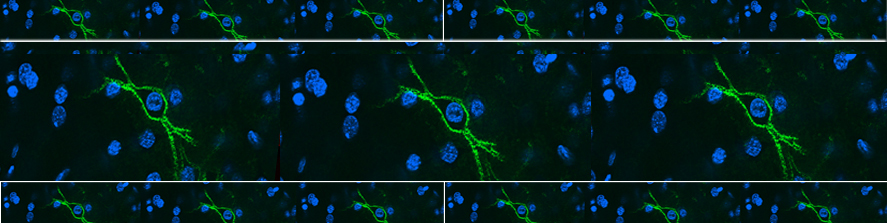
My studies in the De Camilli lab showed that at least three of the four human VPS13 proteins function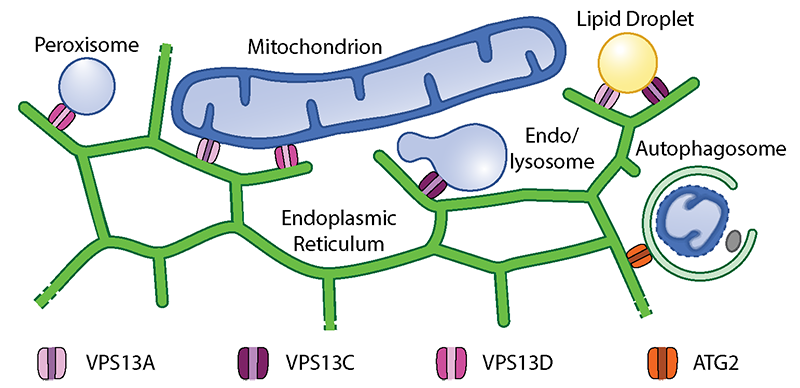 at membrane contact sites. In particular, we discovered that VPS13A and VPS13D acts at mitochondria-ER contacts, and VPS13D is also present at ER-peroxisome contacts, where they can support the crucial exchanges of lipids needed for biogenesis and function of these two organelles. Surprisingly, we found VPS13C, to act instead at the interface between ER and endolysosomes, suggesting slightly different roles of the different VPS13 paralogues (Fig.1).
at membrane contact sites. In particular, we discovered that VPS13A and VPS13D acts at mitochondria-ER contacts, and VPS13D is also present at ER-peroxisome contacts, where they can support the crucial exchanges of lipids needed for biogenesis and function of these two organelles. Surprisingly, we found VPS13C, to act instead at the interface between ER and endolysosomes, suggesting slightly different roles of the different VPS13 paralogues (Fig.1).
Our results together with follow-up studies from our collaborators, showed that VPS13 proteins have a wide and extended, an autophagy protein closely related to the VPS13 family. Such lipid channeling function has no precedent in eukaryotic cells and is well suited to promote the expansion of membrane required for processes such as autophagy and mitochondria biogenesis for which the source of lipids was so far unclear.
Given the implication of each and every VPS13 protein in neurological diseases, our work suggests a fundamental role for lipid dyshomeostasis in neurodegeneration and thus opens the ground for greater understanding of disease pathogenesis.
Future research plans
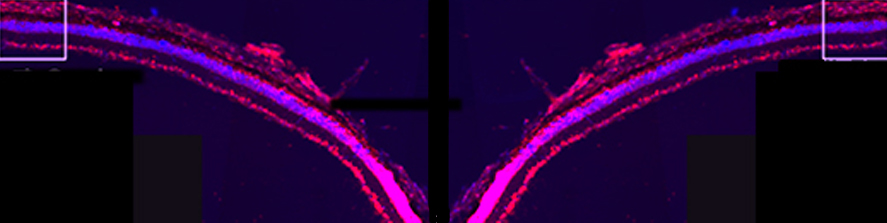
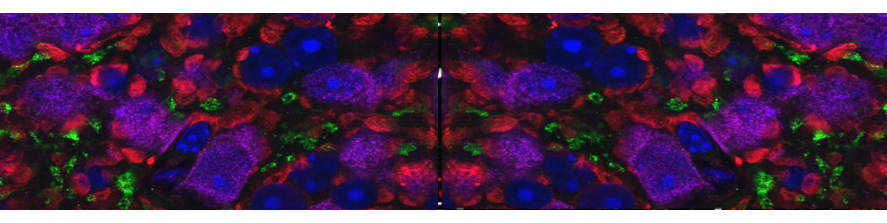
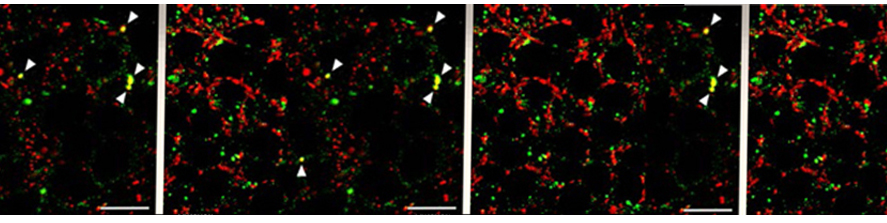
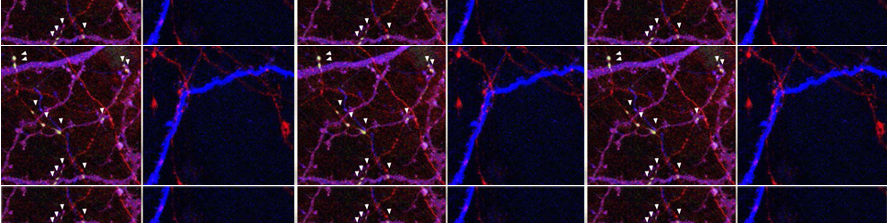
I am going to combine my experience studying the molecular mechanism underlying neuronal pathophysiology with my studies on membrane contact sites and lipid dynamics, shifting the focus on mitochondria and their role in neurodegeneration. Mitochondria are maintained by a finely tuned balance between biogenesis (growth), fission (division), fusion (merging) and mitophagy (a specialized form of autophagy allowing their disposal) (Fig.2). 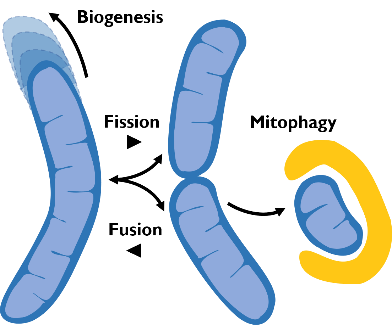 The utmost importance of such balance for neurons, the highest-energy consuming cells in our body, is underscored by the neurological disorders caused by mutations in key components of these processes. Many crucial players of mitochondrial homeostasis have been elucidated, but novel and more selective quality control mechanism, as well as neuro-specific pathways, are just beginning to be uncovered. The contribute of such pathways to neuronal health is an exciting new avenue of investigation that I aim to lead forward with my future research. Importantly, the contact sites with the ER are emerging as crucial hub that orchestrate mitochondrial dynamics.
The utmost importance of such balance for neurons, the highest-energy consuming cells in our body, is underscored by the neurological disorders caused by mutations in key components of these processes. Many crucial players of mitochondrial homeostasis have been elucidated, but novel and more selective quality control mechanism, as well as neuro-specific pathways, are just beginning to be uncovered. The contribute of such pathways to neuronal health is an exciting new avenue of investigation that I aim to lead forward with my future research. Importantly, the contact sites with the ER are emerging as crucial hub that orchestrate mitochondrial dynamics.
The prominent role of mitochondrial homeostasis in neurological diseases, such as Parkinson’s or ataxia, makes this line of research extremely impactful. My research will exploit hiPSCs-derived neurons as the main model system and cutting-hedge microscopy, genome editing, cell biology and biochemistry as experimental approaches.
Representative publications
- Leonzino, et al. Insights into VPS13 properties and function reveal a new mechanism of eukaryotic lipid transport. Biochim Biophys Acta 2021 (under review)
- Guillén-Samander, Leonzino, et al. VPS13D bridges the ER to mitochondria and peroxisomes via Miro. J Cell Biol 2021 (in press)
- Ugur, Hancock-Cerutti, Leonzino and De Camilli. Role of VPS13, a protein with similarity to ATG2, in physiology and disease. Curr Opin Genet Dev 2020
- Kumar, Leonzino, et al. VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J Cell Biol 2018
- Leonzino, et al. The Timing of the Excitatory-to-Inhibitory GABA Switch Is Regulated by the Oxytocin Receptor via KCC2. Cell Rep 2016